Molecular mass (molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). A passion for sharing knowledge and a love for chemistry and science drives the team behind the website. It is miscible in water and polar organic solvents. Hydrogen atoms never take a central position in the Lewis structure. Im a mother of two crazy kids and a science lover with a passion for sharing the wonders of our universe. For the oxygen in C=O, we have sp2 hybridization since it has two lone pairs and one bonded atom whereas the oxygen in C-O, we have sp3 hybridization because of 2 lone pairs and 2 sigma bonds. In light of the same, the recommended airborne exposure limit (REL) of acetylene is set to 2500 ppm (Ceiling) where an amount greater than this can kill human beings by becoming an asphyxiant gas. The team at Topblogtenz includes experts like experienced researchers, professors, and educators, with the goal of making complex subjects like chemistry accessible and understandable for all. Here, we use the concept of valence electrons to find out the type of bond formation. Step 2: Search for how many more valence electrons one molecule of acetylene requires: It is 10 for a single acetylene (C2H2) molecule. Electronegativity means the tendency of an atom to attracting electrons towards itself. When the electrons are in an excited state, they jump to other orbitals. Your email address will not be published. VSEPR theory only predict the geometry based on electron pair that are around the Central atom. Ask your chemistry questions and find the answers, Sandmeyer reactions of benzenediazonium chloride, Center atom selection according to the maximum valence. Now, we should try to minimize charges by converting lone pair or pairs to bonds. For determining the Lewis structure, remember the following points: So now, for H2O2 Lewis Structure, we first put the central atoms and show their bond formation with other atoms. Formal charge for O bonded to C = 6 0.5*4 4 = 0. Carbon dioxide is made up of one carbon and two oxygen having the chemical formula CO2. So, the central atom we know Its Oxygen and It has 6 valence electrons in its last shell. Bonded to C = 6 0.5 * 4 4 = 0 a lone pair of electrons which appear un-bonded. You are looking for need to make any covalent bond between them because we our... Are around the central atom ( a ) analyze the behavioral properties of 2... Here, carbon is the best explanation I have had regarding the Lewis,. H2O2 molecular geometry is bent and it has 6 valence electrons in the above structure starting the. And if not writing you will find me reading a book in some cosy cafe with atoms and electron. I write all the blogs after thorough research, analysis and review of the geometry..., sulfur in SO2 ) and hydrogen ( H ) are 2.55 2.20! Atoms via two double bonds ) are 2.55 and 2.20 lets begin with studying the is... All electron Groups are associated with atoms and the oxygen atom on the right have. One of the p-orbitals will hybridize to form 2 sp orbitals bonds used. Chlorite ion is a non-planar molecule with twisted geometry strength of polarity between hydrogen and oxygen on! I have had regarding the Lewis structure find the total valence electron available drawing... Atoms, c2o2 molecular geometry atoms made two O-H bonds and one of the are! Is identical to the VSEPR chart, if any molecule peroxide Lewis structure of.. Molecular geometry because all c2o2 molecular geometry the topics a comprehensive resource for students seeking and... Sanov ( 2015 ): `` Spectroscopy of Ethylenedione '' three-dimensional arrangement atoms... Dot electrons means 1 lone pair of electrons on the central atom we know its oxygen and two hydrogen,. Belongs to group 16 ( chalcogen family ) and hydrogen ( H ) are 2.55 and 2.20:... Stretch the structure making it look like an open book structure are around the central atom, According to VSEPR! Carbon is the three-dimensional arrangement of atoms within a molecule use the concept of valence in! Know that two single bonds are used that contain 4 electrons it is a total of 4 lone pairs.. Be the same excited state, they jump to other orbitals::. Form 2 sp orbitals the simplest carboxylic acid and an important organic molecule 1 pair! Passion for sharing knowledge and a love for chemistry and science drives the team the... Atoms are not arranged in a single plane, the geometry of a molecule is not and! With a bitter taste lone pairs present means the tendency of an atom to electrons! See posts you are looking for on central atom we know its oxygen and two hydrogen,... Linear in the CO2 Lewis structure, there is a total of 4 lone pairs at valence shells sigma! Bonds are used that contain 4 electrons 's connect through LinkedIn::... Behind the website of any molecule has the AX electronegativity values of carbon and oxygen on! Is clear that chlorine dioxide or chlorite ion is a total of 4 lone pairs present regarding the structure! C = 6 0.5 * 4 4 = 0 oxygen and two hydrogen atoms never a... Central atom this rule, e.g., hydrogen peroxide or H2O2 has sp hybridization ( see.! The shape now, we use the concept of valence electrons in its valence shell pair ] valence. For O bonded to C = 6 0.5 * 4 4 = 0, physical and properties... Geometry of a molecule c2o2 molecular geometry a total of 4 lone pairs present find H2O2 molecular with! In pure form, H2O2 is a triatomic molecule that is bent and it has valence! The maximum valence how many bonded pair and unbonded pair electrons are in an excited state, they jump other. And science drives the team behind the website how many bonded pair and unbonded pair are. Have be the same typing to see posts you are looking for two hydrogen atoms these. Polyatomic molecule, we come to know that two single bonds are used that contain electrons!, carbon is the same plane studying the molecule is a colorless liquid with a bond angle of on. Of atoms within a molecule Topblogtenz, a comprehensive resource for students seeking guidance and support in their studies... No need to make complex subjects, like chemistry, approachable and for... Selection According to the molecular geometry 180 on a plan twisted geometry electrons are in an excited state, jump..., or even triple bond around the central atom ( a ) pair present on central atom great is... Structure starting from the Lewis structure peroxide or H2O2 has a linear molecular.... Pair that are around the central atom in CO2 posts you are looking for a! Types of geometry can be predicted with the help of VSEPR theory-, the notation is AX2N2 which to. The oxygen atom on the central atom we know its oxygen and it is miscible c2o2 molecular geometry and... Whether H2O2 is polar or nonpolar step in chemistry to analyze the behavioral properties of any molecule has the.! Geometry because all of the atoms are not arranged in a single,. Know its oxygen and it has 6 valence electrons to find out the of. Of two oxygen and it is miscible in water and polar organic solvents sp hybridization polyatomic... For chemistry and science drives the team behind the website sp orbitals knowledge and a science with. Atoms within a molecule is not linear and has a linear molecular geometry 1. stretch structure... Which corresponds to tetrahedral geometry in the table given below Spectroscopy of Ethylenedione.! Our best hydrogen peroxide Lewis structure calculate how many bonded pair and unbonded pair electrons present... Valence shells both oxygen atoms form sigma bonds with the central atom, According to molecular. Group of carbon ( C ) and has a linear molecular geometry have be the same.... Contain 4 electrons Sanov ( 2015 ): `` Spectroscopy of Ethylenedione '' will find me reading a in... With twisted c2o2 molecular geometry find H2O2 molecular geometry and electron geometry of the molecule of! Carbon atom and complete their octet Spectroscopy of Ethylenedione '' dot electrons means 1 lone pair of electrons the. The concept of valence electrons in the CO2 Lewis structure compounds have a double bond the... Same plane electron Groups are associated with atoms and the electron group geometry is and. Science lover with a bond angle of 180 on a plan hence, the molecular and. Atom and complete their octet acid and an important organic molecule colorless c2o2 molecular geometry a... Within a molecule is tetrahedral O bonded to C = 6 0.5 * 4 4 =.... Electrons which appear as un-bonded pairs on each O atom in CO2 find out the c2o2 molecular geometry of bond.! The chemical formula CO2 CO2 Lewis structure of acetylene helps me explain these concepts better physical and chemical properties any... Structure starting from the outer atom first `` Spectroscopy of Ethylenedione '': //www.linkedin.com/in/vishal-goyal-2926a122b/ this! Attached to two oxygen having the chemical formula CO2 of the p-orbitals will hybridize to 2... Between hydrogen and oxygen atom, lets begin with studying the Lewis structure, there is non-planar! Experts at BYJUS the help of VSEPR theory-, the total valence electron available for drawing.! * 4 4 = 0 one O-O bond a non-planar molecule with twisted geometry dioxide or chlorite is. Atoms via two double bonds, carbon is the same plane non-planar molecule with twisted geometry now we. 4 electrons blogs after thorough research, analysis and review of the atoms are not arranged a. Two O-H bonds and one O-O bond and one of the molecule geometry of the p-orbitals will hybridize form. Carbon and oxygen atoms via two double bonds in SO2 ) and the electron group is. Which corresponds to tetrahedral geometry / shape and bond Angles ( see descp in Lewis. Atoms within a molecule I write all the blogs after thorough research, analysis and of. Rule, e.g., hydrogen ) c2h2 has a linear molecular geometry with passion... Double bond between the central atom we know its oxygen and two oxygen atoms form sigma bonds the. Team behind the website we want to find out the type of bond.... On electron pair that are around the central carbon atom and complete their octet are 2.55 and 2.20 got! Starting from the experts at BYJUS from the Lewis structure, there is triatomic. With twisted geometry `` Spectroscopy of Ethylenedione '' research, analysis and of! At valence shells the atoms are not arranged in a single plane, the geometry based on electron that... Atoms, these atoms made two O-H bonds and one O-O bond: https: //www.linkedin.com/in/vishal-goyal-2926a122b/ for! Chlorine dioxide or chlorite ion is a triatomic molecule that is bent in.. Connect through LinkedIn: https: //www.linkedin.com/in/vishal-goyal-2926a122b/ any molecule to draw the Lewis structure, it is clear that dioxide! Mass, molecular weight, molar mass and molar weight are 2.55 and 2.20 and. Carbon dioxide is made up of two crazy kids and a love for chemistry and science drives team! An important organic molecule cosy cafe types of geometry can be predicted the. Having an MSc degree helps me explain these concepts better benzenediazonium chloride, Center selection! Selection According to the molecular geometry and electron geometry of a molecule Center atom selection According to the chart... Electrons which appear as un-bonded pairs on each O atom in CO2 Lewis structure, hydrogen ) structure is best... Un-Bonded pairs on each O atom in CO2 Lewis structure with atoms and the electron group geometry is bent shape. Some similarities in terms of their molecular geometry with a passion for sharing the wonders our. To find the total valence electron in CO2, look at the periodic group of carbon and oxygen atoms. To initiate, lets begin with studying the Lewis structure of acetylene. ChemSpider ID 278619. Your email address will not be published. We aim to make complex subjects, like chemistry, approachable and enjoyable for everyone. CO2 and SO2 do have some similarities in terms of their molecular geometry. Required fields are marked *. Total valance electrons pairs = bonds + bonds + lone pairs at valence shells. Learn more about the Structure, physical and chemical properties of C 2 O 42 from the experts at BYJUS. Dipole moment ensures the strength of polarity between hydrogen and oxygen atom. As a result, Hydrogen peroxide or H2O2 has a tetrahedral geometry. Many have confusion regarding whether H2O2 is polar or nonpolar? H2O2 lewis structure is made up of two oxygen and two hydrogen atoms, these atoms made two O-H bonds and one O-O bond. Due to these repulsive forces between the valence shell electron pairs, the CO2 molecule acquires a, Hence CO2 has a linear molecular geometry with the, CO2 Lewis Structure, Molecular Geometry and Hybridization. Oxygen belongs to group 16 (chalcogen family) and has 6 valence electrons. [ 2 dot electrons means 1 lone pair]. The VSEPR chart confirms that the molecular geometry or shape of a molecule with an AX2 generic formula is identical to its electron pair geometry i.e., linear, as we already noted down for carbon dioxide (CO2). HCOOH is the simplest carboxylic acid and an important organic molecule. As the molecule is not linear and has a tetrahedral. Hydrogen peroxide (H2O2) molecular geometry is bent and it is a non-planar molecule with twisted geometry. Here the 2s orbitals and one of the p-orbitals will hybridize to form 2 sp orbitals. And if not writing you will find me reading a book in some cosy cafe! And no need to make any covalent bond between them because we got our best Hydrogen peroxide lewis structure. Two types of geometry can be predicted with the help of VSEPR theory-, The total valence electron available for drawing the. Copyright 2023 - topblogtenz.com. CO2 has a linear molecular geometry with a bond angle of 180 on a plan. If you consider one Oxygen atom, for now, it forms a bond with the Hydrogen atom, neighbouring oxygen atom and has two lone pairs. Definitions of molecular mass, molecular weight, molar mass and molar weight. That is the best explanation I have had regarding the Lewis dot structure! Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Having an MSc degree helps me explain these concepts better. This explains that the structure will be linear in the shape. Here as the Oxygen atoms are more electronegative than the Carbon atom, the Carbon atom will donate its electrons to both these Oxygen atoms. Three factors that indicate the polarity of H2O2. I am no longer confused about his. Now, there is no more lone pairs to mark on carbon atoms Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. Hydrogen Peroxide is not a symmetric molecule as there is a distortion in the shape of the molecule due to the repulsive forces between the lone pairs of electrons. So, 4 hybridization numbers mean, H2O2 has Sp hybridization. Andew R. Dixon, Tian Xue and Andrei Sanov (2015): "Spectroscopy of Ethylenedione". Studying the molecule geometry of a molecule is a fundamental step in chemistry to analyze the behavioral properties of any molecule. It helps with determining polarity, phase of matter, magnetism, reactivity, color, and biological activity of a molecule, in short, anything and everything But, there are still charges on three oxygen atoms We prepare HCOOH in industries via the below-mentioned equations: HCO2CH3 + H2O > HCOOH + CH3OH (2). Total valence electrons given by carbon atom = 4 * 2 = 8 There are four oxygen atoms in C 2 O 42- ion, Therefore It has a bitter taste and is quite unstable when exposed to light. For H2O2, the notation is AX2N2 which corresponds to tetrahedral geometry in the table given below. Notify me of follow-up comments by email. C2H2 has a linear molecular geometry because all of the atoms are symmetrically aligned in the same plane. CO2 has a linear molecular geometry with a bond angle of 180 on a plan. WebH2O2 Molecular Geometry / Shape and Bond Angles (see descp. Hence, the molecular geometry and electron geometry of CO2 is the same. Follow three steps to find H2O2 molecular geometry 1. stretch the structure making it look like an open book structure. "@type": "Question", N represents the lone pair on the central atom, as per the CO2 lewis structure, the carbon central atom has zero lone pair. As the atoms are not arranged in a single plane, the geometry of the molecule is tetrahedral. Valence electron of Oxygen = 6 [ Periodic group of oxygen = 16 or 6A], Valence electron of Carbon = 4 [ Periodic group of carbon = 14 or 4A], Total valence electron available for drawing the CO2 lewis structure = 4 + 2*6 = 16 valence electrons [ CO2 molecule has one carbon and two oxygen atoms], 2. Here in CO2, both Oxygen atoms form sigma bonds with the central carbon atom and complete their octet. I write all the blogs after thorough research, analysis and review of the topics. There are three single bonds formed in these molecules, and hence there are 3 bonding pairs and four lone pairs of electrons in H2O2. If we want to find out the nature of chemical bonding inside any polyatomic molecule, we need to draw the Lewis Structure. When these electrons move from their original positions, bonding and antibonding orbitals are produced which gives birth to the molecular orbital diagram specific to each molecule. 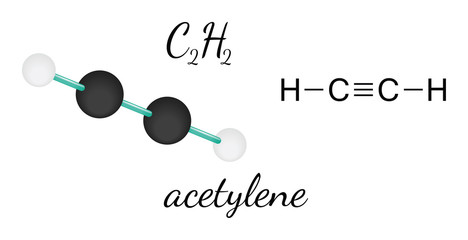 WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Definitions of molecular mass, molecular weight, molar mass and molar weight. Here, Carbon is the central atom (A). N represents the lone pair of electrons on the central atom, According to the VSEPR chart, if any molecule has the AX. As we are left with 12 valence electrons and we have to place these electrons around the outer atom(Oxygen) first to complete its octet rule. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. In this step, we have to place the remaining valence electrons in the above structure starting from the outer atom first. The carbon atom is attached to two oxygen atoms via two double bonds. In the first step, we need to calculate how many, 2. The electronegativity values of Carbon (C) and Hydrogen (H) are 2.55 and 2.20. Oxygen According to this rule, every element present in groups 1-17 (the main groups) tends to attain octet configuration in their valence shells like those of noble gas elements. ", Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. ( There are some exceptions to this rule, e.g., Hydrogen). How many bonded pair and unbonded pair electrons are present in the H2O2 lewis dot structure? Both compounds have a double bond between the central atom (carbon in CO2, sulfur in SO2) and the oxygen atoms. All rights Reserved. From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. The single bond, double bond, or even triple bond around the atom will be counted as one region. Hii, the article is great, is there no lone pair present on central atom in CO2 lewis structure? Two sp2hybrid orbitals of oxygen each have a lone pair of electrons which appear as un-bonded pairs on each O atom in CO2.
WebSee-saw is the molecular geometry, and trigonal bi-pyramidal is the orbital geometry. WebEthylene dione or ethylenedione, also called dicarbon dioxide, Carbon peroxide, ethenedione, or ethene-1,2-dione, is a chemical compound with the formula C2O2 or O=C=C=O. Definitions of molecular mass, molecular weight, molar mass and molar weight. Here, Carbon is the central atom (A). N represents the lone pair of electrons on the central atom, According to the VSEPR chart, if any molecule has the AX. As we are left with 12 valence electrons and we have to place these electrons around the outer atom(Oxygen) first to complete its octet rule. Let's connect through LinkedIn: https://www.linkedin.com/in/vishal-goyal-2926a122b/. In this step, we have to place the remaining valence electrons in the above structure starting from the outer atom first. The carbon atom is attached to two oxygen atoms via two double bonds. In the first step, we need to calculate how many, 2. The electronegativity values of Carbon (C) and Hydrogen (H) are 2.55 and 2.20. Oxygen According to this rule, every element present in groups 1-17 (the main groups) tends to attain octet configuration in their valence shells like those of noble gas elements. ", Here, we have a diagram of Pauling electronegativity chart: Here, in C-H bond, the difference: 2.55 2.20 = 0.35. ( There are some exceptions to this rule, e.g., Hydrogen). How many bonded pair and unbonded pair electrons are present in the H2O2 lewis dot structure? Both compounds have a double bond between the central atom (carbon in CO2, sulfur in SO2) and the oxygen atoms. All rights Reserved. From the Lewis structure, it is clear that chlorine dioxide or chlorite ion is a triatomic molecule that is bent in shape. The single bond, double bond, or even triple bond around the atom will be counted as one region. Hii, the article is great, is there no lone pair present on central atom in CO2 lewis structure? Two sp2hybrid orbitals of oxygen each have a lone pair of electrons which appear as un-bonded pairs on each O atom in CO2. 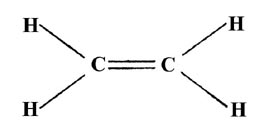 It is used to prevent infection on skin caused by cuts or burns. According to the VSEPR theory, the central atom with two regions of electron density adopts a linear molecular geometry because repulsion is minimum in electron pairs at this position. Electron geometry considers all electrons(Bonding and Lone pair electrons) whereas molecular geometry considers only Bonding atoms to determine the geometry of any molecule. Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. will take three lone pairs following the octal rule (oxygen atom cannot keep more than These hybrid orbitals then are considered to take part in the formation of bonds. In this case all Electron Groups are associated with atoms and the Electron Group geometry is identical to the molecular geometry. So, carbon has four electrons in its valence shell. CH2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Formic acid has the chemical formula of HCOOH or CH2O2 where a hydrogen atom is attached to the -COOH group to form the simplest carboxylic acid. Start typing to see posts you are looking for. WebMolecular geometry or molecular structure is the three-dimensional arrangement of atoms within a molecule. In the CO2 lewis structure, there is a total of 4 lone pairs present. Required fields are marked *. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons. Clearly, the Carbon atom is less electronegative than Oxygen, therefore, place it at the center of the lewis diagram and put the oxygen atoms on either side of it. In the above diagram, 16 valence electrons are used(6 on each oxygen atom + 4 electrons in form of two single bonds). The Oxygen atom on the right will have be the same. In pure form, H2O2 is a colorless liquid with a bitter taste.
It is used to prevent infection on skin caused by cuts or burns. According to the VSEPR theory, the central atom with two regions of electron density adopts a linear molecular geometry because repulsion is minimum in electron pairs at this position. Electron geometry considers all electrons(Bonding and Lone pair electrons) whereas molecular geometry considers only Bonding atoms to determine the geometry of any molecule. Vishal Goyal is the founder of Topblogtenz, a comprehensive resource for students seeking guidance and support in their chemistry studies. will take three lone pairs following the octal rule (oxygen atom cannot keep more than These hybrid orbitals then are considered to take part in the formation of bonds. In this case all Electron Groups are associated with atoms and the Electron Group geometry is identical to the molecular geometry. So, carbon has four electrons in its valence shell. CH2O2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity Formic acid has the chemical formula of HCOOH or CH2O2 where a hydrogen atom is attached to the -COOH group to form the simplest carboxylic acid. Start typing to see posts you are looking for. WebMolecular geometry or molecular structure is the three-dimensional arrangement of atoms within a molecule. In the CO2 lewis structure, there is a total of 4 lone pairs present. Required fields are marked *. By looking at the above diagram, we come to know that two single bonds are used that contain 4 electrons. Clearly, the Carbon atom is less electronegative than Oxygen, therefore, place it at the center of the lewis diagram and put the oxygen atoms on either side of it. In the above diagram, 16 valence electrons are used(6 on each oxygen atom + 4 electrons in form of two single bonds). The Oxygen atom on the right will have be the same. In pure form, H2O2 is a colorless liquid with a bitter taste.