 $$ Cl^{-} + MnO_{4}^{-} \rightarrow Cl_{2}^{o} + Mn^{2+} $$. WebIn reactions involving many compounds, equations can be balanced using an algebraic method, based on solving a set of linear equations. $$ {Mg} \left( s \right) + {Cu(NO_3)_2} \left( aq \right) \rightarrow {Mg(NO_3)_2} \left( aq \right) + {Cu} \left( s \right) $$. Get through the table below that highlights all the symbols that one must encounter when carrying out a chemical reaction in the laboratory. Write correct formulas for the products in these single replacement reactions. WebCalculator designed to balance chemical equations with results of: the balanced equation, word equation, and how it happened. Web1. Disable your Adblocker and refresh your web page . Keep in mind, that to be safe - especially if your teacher is unclear on what to do - you may want to do all possible answers. That is why we will be using fraction coefficient here as pr the temporary rule defined: $$ C_{4}H_{10} + \frac{13}{2} O_{2} \rightarrow 5H_{2}O + 4CO_{2} $$. Step 2: Click Submit to receive the results. The products of this single-replacement reaction are CaCl. wm_track_alt='';
$$ Cl^{-} + MnO_{4}^{-} \rightarrow Cl_{2}^{o} + Mn^{2+} $$. WebIn reactions involving many compounds, equations can be balanced using an algebraic method, based on solving a set of linear equations. $$ {Mg} \left( s \right) + {Cu(NO_3)_2} \left( aq \right) \rightarrow {Mg(NO_3)_2} \left( aq \right) + {Cu} \left( s \right) $$. Get through the table below that highlights all the symbols that one must encounter when carrying out a chemical reaction in the laboratory. Write correct formulas for the products in these single replacement reactions. WebCalculator designed to balance chemical equations with results of: the balanced equation, word equation, and how it happened. Web1. Disable your Adblocker and refresh your web page . Keep in mind, that to be safe - especially if your teacher is unclear on what to do - you may want to do all possible answers. That is why we will be using fraction coefficient here as pr the temporary rule defined: $$ C_{4}H_{10} + \frac{13}{2} O_{2} \rightarrow 5H_{2}O + 4CO_{2} $$. Step 2: Click Submit to receive the results. The products of this single-replacement reaction are CaCl. wm_track_alt='';
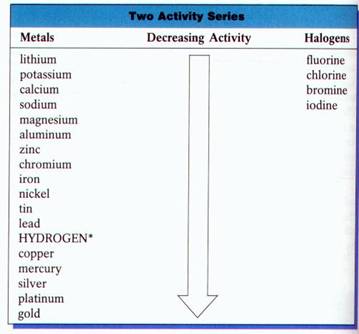
In this reaction scheme, Barium Hydroxide reacts with Copper Thiocyanate to form double products that are Barium Thiocyanate and Cupric Hydroxide. Note that none of the example problems above are balanced. Course Hero is not sponsored or endorsed by any college or university. (If you would like to cover that material now, please refer to this other textbook, which has been adapted for the content in the remainder of this section. Now this is considered a balanced reaction as the number of the atoms on the left and right side are exactly equal. As mentioned at the start of this discussion, we will be adding a 4th step in order to determine whether these reactions will occur in a later chapter. You can instantly balance these reactions by subjecting to this best balancing chemical reactions calculator. (note: if you are unsure how we determined these charges, please refer to the section of this book where we first discussed ionic compounds.) WebThe general form of a single-replacement (also called single-displacement) reaction is: A+ BC AC + B In this general reaction, element A is a metal and replaces element B, WebCalculate chemical reactions step-by-step. Other things to remember for single replacement reactions include: Elements that are likely to form cationsusually metals or hydrogen gaswill replace the cation in a compound, and elements that are likely to form anionsusually group 17 halogenswill replace anions in a compound. Predict what will happen if rubidium hydroxide and cobalt(II) chloride are mixed. However, an online Percent Yield Calculator helps you to calculate the percent yield value by adding theoretical yield and actual yield value. Here is another way to look at the above generic example: Keep in mind that, when it comes to writing actual formulas, you MUST write chemically correct formulas. To embed this widget in a post on your WordPress blog, copy and paste the shortcode below into the HTML source: To add a widget to a MediaWiki site, the wiki must have the. You need to be able to recognize single replacement reactions AND be able to break a formula apart into proper cations and anions as well as write correct formulas. Balance the following chemical equation given as under: $$ Zn + HCl \rightarrow ZnCl_{2} + H_{2} $$. That is, a metal will replace a metal and a non-metal will replace a nonmetal. (Zn has a charge of +2. Lets move forward! You can better understand it with the help of this balancing reactions calculator. A simple equation that represents a single-replacement reaction is: AB + C -> A + BC Single-replacement reactions always involve two pure elements and one aqueous compound/solution.